Which of Our products approved by Canada Health?
In Canada, the sale and distribution of medical devices are strictly regulated by Health Canada to ensure patient safety and product efficacy.
One of the key requirements for manufacturers is obtaining a Medical Device Licence (MDL), particularly for Class II, III, and IV medical devices.
What is an MDL (Medical Device Licence)?

An MDL (Medical Device Licence) is an official authorization issued by Health Canada, permitting a company to legally sell its medical device in the Canadian market.
The requirement for an MDL depends on the risk classification of the device:
Class I (Low Risk) – Examples include bandages, thermometers, and non-invasive devices. These do not require an MDL but must still comply with general safety regulations.
Class II (Medium Risk) – Includes devices like syringes, contact lenses, and some dermatological tools. These require an MDL and must meet additional safety and performance standards.
Class III (High Risk) – Covers devices such as surgical lasers, implantable devices, and certain advanced medical equipment. These undergo rigorous review before approval.
Class IV (Highest Risk) – Includes life-sustaining devices like pacemakers, heart valves, and other critical implants. These face the strictest regulatory scrutiny.
Since microneedling devices are classified as medical instruments that penetrate the skin, they typically fall under Class II or higher, meaning they must obtain an MDL before being sold in Canada.
Dr.Pen’s Commitment to Compliance & Innovation

At Dr.Pen, we prioritize safety, efficacy, and regulatory compliance in all our products.
Due to the growing demand for professional microneedling devices in North America, our R&D team has developed advanced models specifically engineered to meet Canada’s stringent medical device standards.
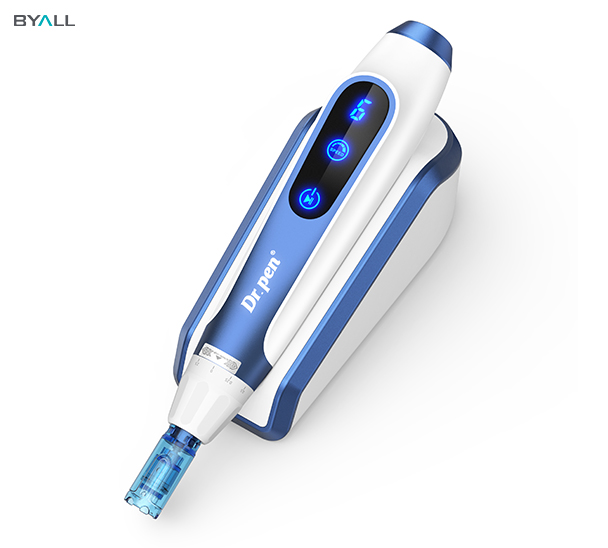
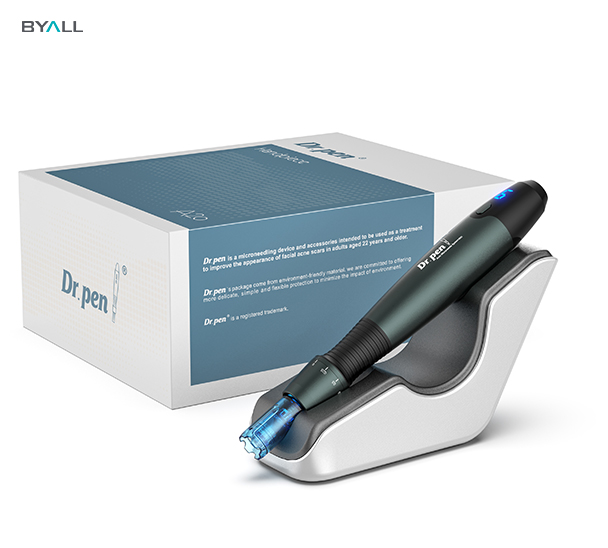
Dr.Pen A9 ,Dr.Pen A11 & Dr.Pen A20: MDL-Certified Microneedling Devices
Our Dr.Pen A9 and Dr.Pen A20 are designed with cutting-edge technology to ensure optimal performance, safety, and user satisfaction.
These models have been meticulously crafted to comply with Canadian MDL requirements, making them a trusted choice for both professionals and at-home users.
Key Features of Dr.Pen A9 ,Dr.Pen A11 & Dr.Pen A20
✅ 3-Snap Fixed Needle Structure – Ensures maximum stability and precision during treatments, reducing the risk of uneven penetration.
✅ Wavy Contact Surface Technology – Enhances serum absorption by creating micro-channels that allow skincare products to penetrate deeper into the skin.
✅ Upgraded 18-Needle Cartridge (0.18mm Diameter) – Delivers faster skin rejuvenation with minimal trauma, promoting quicker recovery and better results.
✅ Medical-Grade Materials – Manufactured under strict quality control to meet Health Canada’s safety standards.
✅ Adjustable Speed & Depth – Allows for customizable treatments tailored to different skin types and concerns.
Why Choose an MDL-Certified Microneedling Device?
Purchasing a Health Canada-approved microneedling device ensures:
✔ Safety – The device has undergone rigorous testing for biocompatibility, electrical safety, and performance.
✔ Effectiveness – Clinically validated technology that delivers real results for collagen induction, scar reduction, and skin rejuvenation.
✔ Legal Compliance – Avoids regulatory issues, ensuring that the device can be sold and used without restrictions in Canada.
Experience Professional-Grade Microneedling with Dr.Pen
Whether you’re a skincare professional or an at-home beauty enthusiast, the Dr.Pen A20 provide medical-grade performance with the convenience of safe, FDA-cleared (U.S.) and MDL-certified (Canada) technology.
✨ Upgrade Your Skincare Routine with Dr.Pen – Where Innovation Meets Compliance! ✨
For more details on certifications and product specifications, visit our website or contact our customer support team.