What is the FDA?
For those encountering the FDA for the first time, a common question arises: What does FDA stand for? The FDA is an abbreviation for the U.S. Food and Drug Administration, a governmental regulatory body in the United States, akin to China’s CFDA (China Food and Drug Administration).

The full English name of the U.S. Food and Drug Administration is U.S. Food and Drug Administration, commonly referred to as the FDA.
The primary responsibility of the U.S. FDA is to oversee the anti-terrorism registration and traceability of products entering the U.S. market, including food, feed, drugs, medical devices, cosmetics, and more.
It ensures the safety of food, cosmetics, drugs, biological preparations, medical equipment, and radiation products produced domestically in the United States or imported into the country.

This necessitates that relevant products imported into the U.S. undergo FDA registration, filing, or testing and certification to ensure their safety.
Food-related enterprises are often the ones most frequently required to engage with the FDA. When exporting products to the U.S. market, these companies need to complete FDA registration, obtaining an 11-digit registration number.
This number must be provided to customs for clearance into the U.S. market.
Additionally, the FDA is responsible for issuing regulations regarding food-grade materials. Third-party laboratories with relevant laboratory qualifications can conduct tests according to FDA regulations and issue FDA test reports.
Currently, products entering the U.S. market are subject to different FDA certification requirements based on their categories, mainly including FDA testing and FDA registration.
What Inspections Are Required for Microneedling Products to Obtain FDA Certification?
In the era of the rising “beauty economy” and the prevalence of light medical aesthetics, microneedling pens have gained significant popularity among beauty enthusiasts due to their remarkable effects in shrinking pores, reducing wrinkles, and tightening the skin.
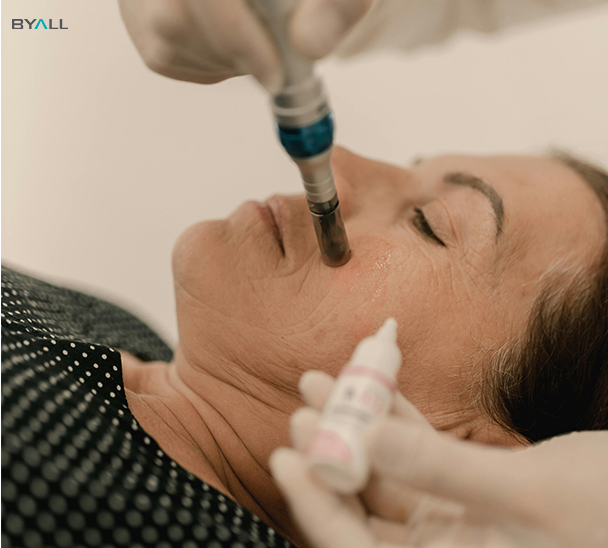
According to industry data, the market size of China’s microneedling industry surged from 4.502 million yuan in 2018 to 7.277 million yuan in 2022.
The global market for gold microneedling radiofrequency devices reached 550 million U.S. dollars in 2022 and is projected to double to 1.25 billion U.S. dollars by 2030.
Behind this enormous market potential lies a stringent demand for product safety and efficacy.
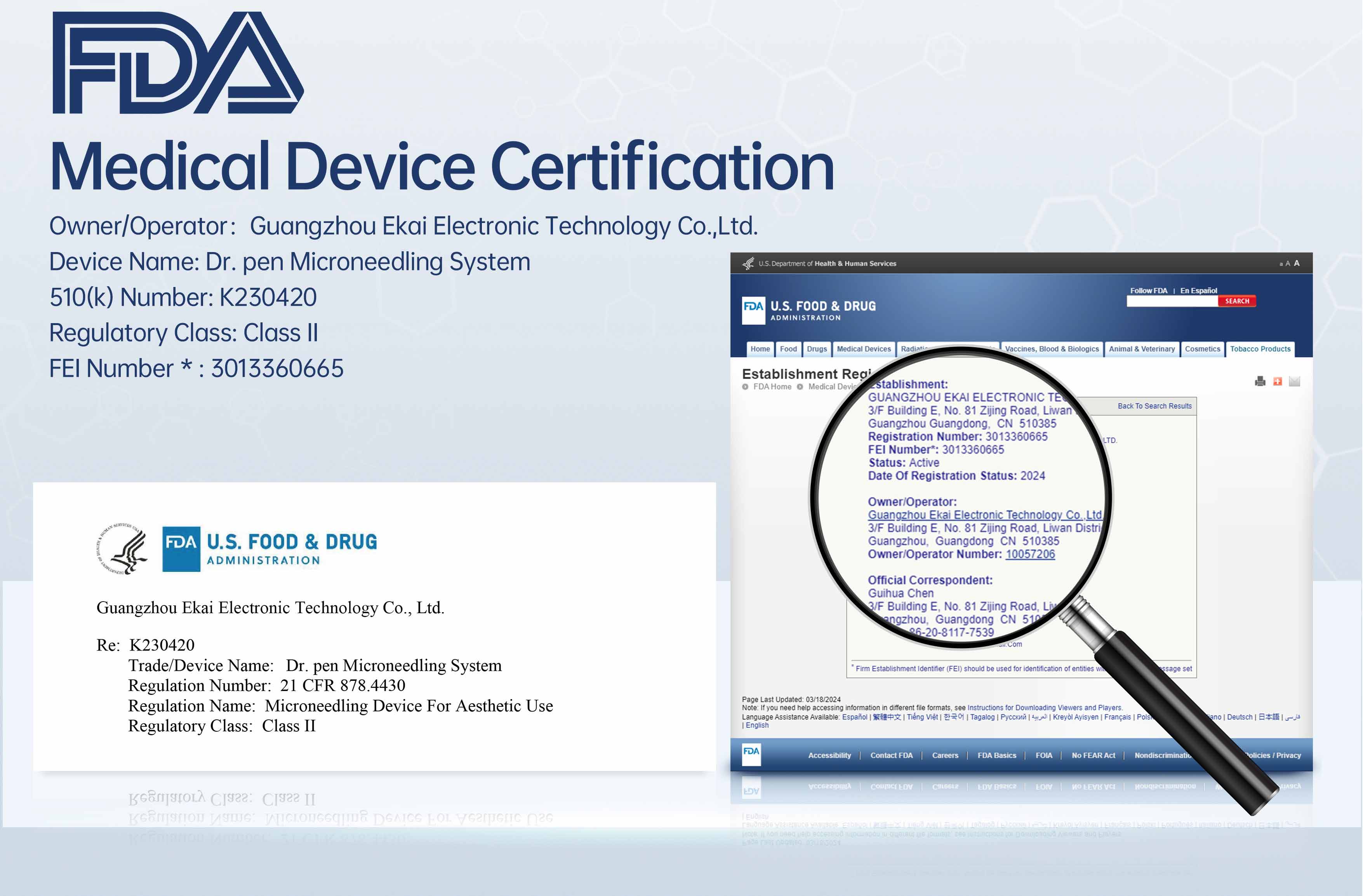
The FDA 510(k) certification serves as the crucial “key” to entering the high-end market in the United States and even globally.
However, this certification process involves complex technical and regulatory requirements, posing significant challenges for enterprises.
Next, we will delve into the inspections that microneedling products must undergo to obtain FDA certification.
1. Comprehensive Performance Testing
Performance testing is a critical step in ensuring the safety and efficacy of radiofrequency microneedling devices, encompassing several key testing aspects:
●Radiofrequency Output Stability
This involves verifying the accuracy of energy output. For instance, temperature control must be precise within ±1°C, and impedance matching should fluctuate within specified ranges.
●Consistency of Treatment Depth
During treatments on different body parts, the depth deviation should not exceed ±0.1mm.
●Microneedle Mechanical Performance Testing
This is equally indispensable.
Needle strength must withstand a certain amount of pressure without breaking.
The repeatability of penetration depth should be controlled within ±0.05mm.
Fatigue testing simulates the deformation of microneedles after multiple uses to ensure stable performance within the specified number of uses.
●Biocompatibility
According to ISO 10993-1 standards, the cytotoxicity, sensitization, and irritation of components in contact with the skin are evaluated.

If microneedles penetrate the dermis layer, additional considerations for chronic toxicity are necessary due to long-term contact with human tissues, necessitating strict safety controls.
2. Sterilization and Shelf-Life Validation
●Sterilization Verification for Sterile Products
For products provided as sterile, such as disposable microneedle tips, the sterilization process must be validated.
For example, the residual amount of EO (ethylene oxide) sterilization should be controlled within safe limits, generally below specified ppm values, to prevent harm to the human body.
●Validation of Cleaning and Disinfection Methods for Reusable Products
For reusable products, the effectiveness of cleaning and disinfection methods must be verified to ensure that the device can effectively remove microorganisms and contaminants after each use, guaranteeing safety for subsequent uses.
●Shelf-Life Validation
This typically involves real-time aging tests. Simultaneously, a sterilization process validation report (such as ISO 11135) should be provided, detailing sterilization process parameters, equipment operation status, and biological indicator test results to prove the effectiveness and stability of the sterilization process.
3. Clinical Data Requirements

Enterprises need to prepare comprehensive product documentation, including technical principles, performance test data, and comparative analyses with similar devices, to thoroughly explain the product’s characteristics and advantages to FDA experts and understand the FDA’s specific expectations for the data.
Additionally, experts will assess whether clinical data can be supplemented or replaced by literature data (such as published studies on the efficacy of radiofrequency microneedling).
If there is high-quality literature supporting the product’s safety and efficacy, enterprises can propose this to the FDA during communication to seek recognition.
Why Choose FDA-Certified Microneedling Devices?
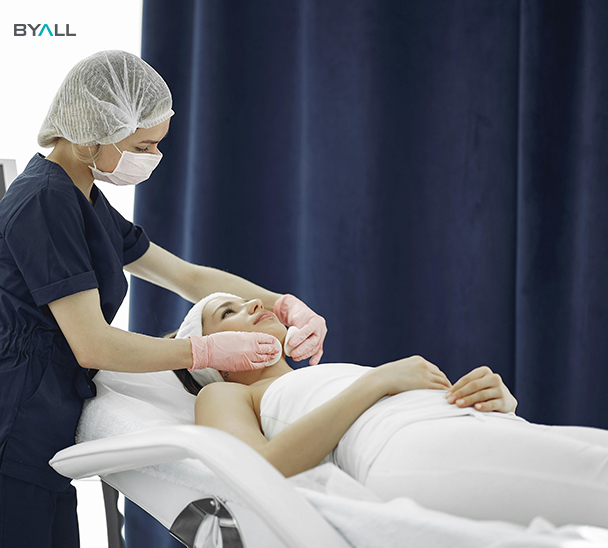
1. Safety
●Stringent Testing
FDA-certified microneedling devices have undergone rigorous biocompatibility, electrical safety, and performance testing, ensuring they are safe for use.
2. Efficacy
●Clinically Validated Technology
These devices utilize technologies that have been clinically proven to be effective in collagen induction, scar reduction, and skin regeneration.
3. Compliance
●Avoiding Regulatory Issues
Choosing FDA-certified devices ensures that they can be sold and used without restrictions in the United States, avoiding potential regulatory complications.
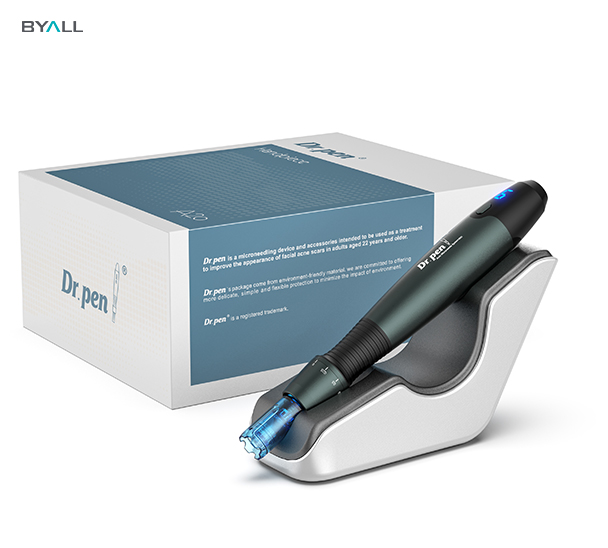
Irreplaceable Advantages of FDA-Certified Microneedling Pens (Taking Dr. Pen as an Example)
1. Lightweight and High-Quality Materials
Dr. Pen microneedling pens are crafted from lightweight, high-quality materials. They are not only durable but also feature an ergonomic grip design, enabling convenient and quick at-home microneedling treatments.
2. Affordable Pricing
Compared to other microneedling pens, Dr. Pen offers more affordable pricing. With its lightweight operation, diverse product range, and comprehensive functions, it can meet various skin needs.
Whether in beauty clinics or for home treatments, Dr. Pen provides convenient and professional microneedling solutions.
3. Excellent Product Design
●Wave-Shaped Needle Contact Surface
The latest needles feature a wave-shaped contact surface, enhancing the absorption of active ingredients and avoiding the waste of essence.
●Three-Snap Fixing Structure
This structure ensures greater stability and safety in performance.
●Innovative 14-Needle Design
With a diameter of 0.18mm, compared to the common 0.25mm on the market, it causes less pain, delivers better treatment effects, results in less damage, and allows for faster recovery.
By choosing Dr. Pen, you can enjoy an economical, effective, and user-friendly microneedling experience that seamlessly integrates into your daily skincare routine.

Whether you are a professional aesthetician or a home skincare enthusiast, Dr. Pen is the ideal choice.
For more information on our microneedling pens, visit the Dr. Pen comparison page or contact our knowledgeable customer service.
For the Dealers and Distributors, please don’t hesitate to contact us to get a direct factory price today!